In June 2018, the first healthy term infant was enrolled in the VOYAGE clinical study. The official title of this study is: A Randomised, Controlled, Double-blind, Parallel Group, Multi-country Study to Investigate the Safety and Tolerance of a Partly Fermented Infant Formula Containing Prebiotic Oligosaccharides in Healthy Term Infants. VOYAGE is starting in Belgium, Spain, Ukraine, Hungary and Poland.
The study is registered at www.clinicaltrials.gov under NCT number: NCT03476889. More details can be found here.
The overall objective of VOYAGE is to study the safety and tolerance of a next generation infant formula. To be consistent with new European regulation on the composition of infant formula per 2020, this new formula combines our safe and extensive researched postbiotics (bioactive compounds produced during the Lactofidus™ fermentation process) and our unique mix of prebiotic fibres in healthy term infants.
The primary objective of the study is to test equivalence of weight gain per day from baseline until the age of 17 weeks in subjects receiving the new product compared to subjects receiving the control product. Additionally, data on other anthropometric parameters, safety and tolerance parameters and investigator-reported adverse events (AEs) is collected.
Human milk is our gold standard
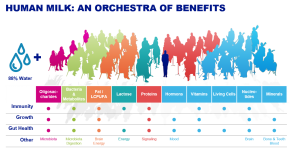
It is universally accepted that the best form of nutrition for infants beginning at birth is human milk.1WHO. The World Health Organization’s infant feeding recommendation. who.int. Published on 2017. Accessed 2018 http://www.who.int/nutrition/topics/infantfeeding_recommendation/en/. Human milk provides a complete supply of nutrients to support growth and development in early life, as well as bioactive compounds that beneficially affect intestinal health, gut microbial colonisation and immune maturation.2Field C, The immunological components of human milk and their effect on immune development in infants. J Nutr. Published on. Published on 2005;135(1):1-4 ,3Field C The immunological components of human milk and their effect on immune development in infants. J Nutr.Published on. Published on 2005;135(1):1-4 When a mother is unable to, or chooses not to breastfeed, infant formula is the best alternative. It is important that formula-fed infants receive the most scientifically advanced and nutritionally beneficial formulae available. Research to improve the quality of infant formulae aims to mimic the composition of human milk as much as possible and, above all, to achieve the functional effects that are observed in breastfed infants.4Aggett P, Agostini C, Goulet O, et al. The nutritional and safety assessment of breast milk substitutes and other dietary products for infants: a commentary by the ESPGHAN Committee on Nutrition. J … Continue reading
VOYAGE Methodology and intervention
The study population consists of healthy, term, singleton infants. Approximately 200 infants aged ≤14 days, whose parent(s) have autonomously decided to exclusively formula feed, will be randomised to receive either the new product or the control product. A breastfed reference arm will be enrolled, with 60 subjects whose mothers intend to exclusively breastfeed until at least the age of 17 weeks. These subjects will not be randomised. Recruitment is expected to be completed in June 2019.
More information on fermentation can be found here
View References
| 1 | WHO. The World Health Organization’s infant feeding recommendation. who.int. Published on 2017. Accessed 2018 http://www.who.int/nutrition/topics/infantfeeding_recommendation/en/. |
|---|---|
| 2 | Field C, The immunological components of human milk and their effect on immune development in infants. J Nutr. Published on. Published on 2005;135(1):1-4 |
| 3 | Field C The immunological components of human milk and their effect on immune development in infants. J Nutr.Published on. Published on 2005;135(1):1-4 |
| 4 | Aggett P, Agostini C, Goulet O, et al. The nutritional and safety assessment of breast milk substitutes and other dietary products for infants: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr. Published on 2001;32(3):256-258 |