Results of the LIFE study presented at the 2017 Excellence in Pediatrics conference
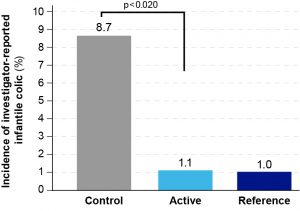
That was one of the findings of the LIFE study, presented by drs. H. Bouritius, senior scientific program leader at Danone Research & Innovation at the EIP conference in Vienna. The investigators reported fewer cases of infantile colic in these healthy term-born infants receiving the partly fermented infant formula combined with scGOS/lcFOS (1.1%) compared to the control group (8.7%) (P=0.020, Fisher’s exact test). Infantile colic was reported 1.0% in the breastfed reference group (Figure).
LIFE study
The LIFE (Prebiotic oLIgosaccharides and FEmented formula study) is a prospective randomised, double blind, controlled multicentre trial in which we investigate the effects of an infant formula combining a specific fermented milk powder (unique fermentation process; LactofidusTM) with prebiotic oligosaccharides scGOS/lcFOS (0.8 g/100 ml, 9:1) on gastrointestinal related parameters. Previously, stool data (showing softer, more frequent stools in the intervention group) was published by Herrera et al.1Herrera AR, Ludwig T, Bouritius H, et al. OP-18 THE COMBINATION OF SCGOS/LCFOS AND FERMENTED INFANT FORMULA SOFTENS STOOLS OF INFANTS COMPARED TO UNFERMENTED INFANT FORMULA WITHOUT SCGOS/LCFOS. J. … Continue reading,2Rodriguez-Herrera A, Ludwig T, Swinkels S, The systematic effect of infant formulas on stool consistency proven by longitudinal statistical models. JPGN.. Published on 2016;62:422 Furthermore, a partly fermented formula with scGOS/lcFOS has also been reported to prevent infantile colic (derived from parent-reported crying duration) at 4 weeks of age.3Vandenplas Y, Ludwig T, Bouritius H, del. Randomised controlled trial demonstrates that fermented infant formula with short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides … Continue reading
Study set up
Healthy formula fed infants aged ≤ 28 days (n=200) were randomised to receive either the partly fermented formula with scGOS/lcFOS (active) or a non-fermented IF without scGOS/lcFOS until 17 weeks of age (control). An exclusively breastfed group (n=100) was included as reference. The incidence of adverse events including infantile colic was reported by the investigators. Crying duration and frequency data were collected daily with a modified Baby Day Diary for the entire intervention period (Trial registration number NTR3455).
All abstracts from EIP, including this one, can be found here.
View References
| 1 | Herrera AR, Ludwig T, Bouritius H, et al. OP-18 THE COMBINATION OF SCGOS/LCFOS AND FERMENTED INFANT FORMULA SOFTENS STOOLS OF INFANTS COMPARED TO UNFERMENTED INFANT FORMULA WITHOUT SCGOS/LCFOS. J. Published on 2015;61(4):516-517. doi 10.1097/01.mpg.0000472222.09292.b9 |
|---|---|
| 2 | Rodriguez-Herrera A, Ludwig T, Swinkels S, The systematic effect of infant formulas on stool consistency proven by longitudinal statistical models. JPGN.. Published on 2016;62:422 |
| 3 | Vandenplas Y, Ludwig T, Bouritius H, del. Randomised controlled trial demonstrates that fermented infant formula with short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides reduces the incidence of infantile colic. Published on 2017;106(7):1150-1158. doi 10.1111/apa.13844 |