Title: Tolerance development in cow’s milk allergic infants receiving amino-acid-based formula
| Authors: | P. Chatchatee et al |
| Published: | 2021 |
| Journal: | The Journal of Allergy and Clinical Immunology |
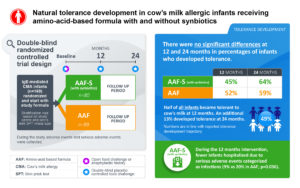
The PRESTO study is the first double blinded randomized-controlled clinical study to investigate the trajectory of cow’s milk (CM) tolerance development and safety of 12 months use of an amino-acid-based formula (AAF) containing synbiotics (AAF-S), consisting of prebiotic-oligosaccharides scFOS/lcFOS and probiotic Bifidobacterium breve M-16V. Infants under 13 months with confirmed IgE-mediated CM allergy (CMA), including those with a history of anaphylaxis and multiple food sensitizations, were randomized to receive AAF-S (n=80) or AAF (n=89) for 12 months, with follow-up for an additional 2 years.
The study was conducted at 20 sites in 6 countries (Germany, Italy, Singapore, Thailand, United Kingdom, and United States of America).
Marleen van Ampting, Project Leader Allergy Treatment at Danone Research & Innovation:
“The development of tolerance is important for the health and quality of life of infants suffering from food allergies and their families. We developed the PRESTO study which, to our knowledge, is the first randomized controlled study to show the natural tolerance development in infants with confirmed IgE-mediated CMA receiving an AAF with or without synbiotics.”
The PRESTO study results show that AAF-S is safe and suitable for dietary management of infants with IgE-mediated CMA. Whilst there were no statistically significant differences in CM tolerance development observed between the groups, 49% of all infants became tolerant to CM at 12 months and an additional 13% developed tolerance at 24 months. This new clinical evidence shows that these infants using specific amino acid formulations develop tolerance to CM at a similar trajectory compared to various cohorts and interventions with different hypoallergenic formula. These results are clinical importance for the CMA management decisions in infants with IgE-mediated CMA or multiple food allergies.
Another important finding was that during the 12-months intervention fewer infants receiving AAF-S were hospitalized due to serious adverse events categorized as infections.
The study is published in the Journal of Allergy and Clinical Immunology.
Background
The global prevalence of allergy is steadily rising, with up to 40% of the world’s population now affected by one or more allergic conditions. CMA is one of the most common food allergies in infancy.
Allergy is an unwanted reaction of the immune system and can be either immunoglobulin E (IgE) or non-IgE mediated.
In IgE mediated CMA the immune system reacts to CM protein by producing IgE antibodies, resulting in gastrointestinal, respiratory, and/or skin symptoms. These symptoms typically occur immediately after ingestion of cow’s milk protein, whereas non-IgE mediated reactions, which mostly result in gastrointestinal or skin symptoms, usually appear after 48 hours or more. In severe cases, an IgE-mediated allergic reaction could lead to anaphylaxis — a potentially life-threatening allergic reaction that comes on quickly, affects the whole body, and requires medical help straight away
While there’s no cure, some children outgrow their CMA as they get older. The point in time to develop tolerance to CM is known to dependent on the type of CMA and is notably slower, thus more persistent, for children with IgE-mediated compared with non-IgE-mediated CMA. Therefore, well designed clinical studies investigating the CMA trajectory, especially of IgE-mediated CMA, are needed.
Current knowledge indicates that 70-80% of immune cells are located in the gut, and the immune system development relies on establishing a balanced and diverse community of microbes (gut microbiota) in early life. Therefore, nutrition during early life is an opportunity to impact the gut microbiota and subsequent immune system development.
Human milk (breastfeeding) is always the best source of nutrition for infants and is known to contain compounds, such as prebiotic oligosaccharides and beneficial bacteria, that support the gut microbiota development.
When breastfeeding is insufficient or not possible infants with CMA may need a suitable hypoallergenic formula, such as an extensively hydrolysed formula (eHF) or an AAF. An AAF is indicated in more severe cases of CMA, for example in situations of anaphylaxis, multiple food allergies, growth faltering, or when an eHF is not tolerated.
Clinical evidence has associated a so called dysbiosis of the gut microbiota in infants with CMA, as lower levels of bifidobacteria have been found compared to the microbiota of healthy breastfed infants. This observation and the key role of the gut microbiota in immune system development, led to the rationale to supplement hypoallergenic formulas for these infants with pre-, pro- and synbiotics (a combination of prebiotics and probiotics), suitable for use in CMA, to target the gut microbiota dysbiosis and to subsequently support the immune system development.
Previous studies have shown that a specific AAF-S is hypoallergenic, supports normal growth and improves the gut microbiota when used as a sole source of nutrition in healthy, full-term infants, and in infants with IgE- or non-IgE-mediated CMA.
However, the effect of this specific AAF and AAF-S on CM tolerance development trajectory had not been studied before.